|
1.
Remove lids of TTX plates and dry the plates for ~ 60 min at
room temperature. |
 |
|
2. Place the TTX plates with lids upside-down and mark center and a point of 1.0 ~ 1.5 cm from edge. |
 |
|
3.
Take the vials with frozen glacial acetic acid out from cold
room and leave the vials for ~ 10 min at room temperature
(25 Ž¡C) to allow acetic acid starting to melt |
 |
|
4.
Put the vial on the center of TTX plate. |
 |
|
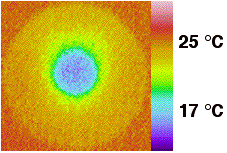
5.
Leave for 10 ~ 15 min to form stable radial thermal gradient
on the surface of TTX plate. |
 |
|
6.
Remove the vial and place an animal on the point of TTX
plate. |
 |
|
7. Put the vial on the center of TTX plate again |
|
|
8.
Repeat step 6 and 7 for each TTX plate ¡¡ beginner: 20 min skillful: 10 min expert: 6 min |
 |
|
9.
Leave for 50 ~ 60 min. |
|
|
10. Remove the vial and put several drops of chloroform on the lid to stop the animal |
 |
|
11. Place the TTX plates at 4 Ž¡C until photograph |